Three-component aviation kerosene model fuel combustion research progress
Aviation kerosene is the "heart pacemaker" of aviation engines. Carrying out combustion research on aviation kerosene can on the one hand reveal the mechanism of aviation fuel combustion and improve the combustion efficiency; on the other hand, it can effectively regulate the emission of pollutants through the reaction path. However, the actual aviation kerosene contains hundreds of components and contains a large amount of isomers, and the reaction mechanism is very complicated. It is very difficult to consider the kinetic mechanism of each component. Researchers often choose typical components to form model fuels, so that they exhibit physical and chemical properties similar to actual aviation fuels. Model fuel for actual aviation fuel.
Recently, researchers from the New Technology Laboratory of the Institute of Engineering Thermophysics of the Chinese Academy of Sciences have responded to n-dodecane (66.2%), n-propylbenzene (15.8%) and 1,3,5-trimethylcyclohexane (18.0%) The combustion kinetics of the three-component aviation model fuel was studied. The fuel composition (H / C ratio and molar mass) of this model is similar to Jet-A and RP-3 aviation fuels. Therefore, it is feasible to use this model fuel to predict and simulate actual aviation kerosene. The researchers conducted an oxidation test under the experimental conditions of P = 1atm, T = 575-1100K, Φ = 2 using a jet-stirred reactor, and obtained the species mole fraction curve (Figure 1).
In addition, during the visit of the German Aerospace Center, Dr. Yuexi Liu of the Institute of Engineering Thermophysics conducted a high-pressure (P = 16bar) shock tube experiment with a T = 700-1500K and an equivalence ratio of 1 to obtain the ignition delay time of the fuel and use it The flame propagation velocity of the laminar flow furnace was measured under the experimental conditions of T = 473K, P = 1atm, Φ = 0.6-2.0. According to the experimental measurement results and the quantum chemical calculation method, important initial reaction rate constants are obtained, and a detailed chemical kinetic mechanism including 401 components and 2838 reactions is established. The reaction mechanism can well simulate the mole fraction curve, ignition delay time and flame burning speed obtained in the experiment. The NTC effect observed in the low-temperature oxidation and high-pressure shock tube experiments is also very good through this mechanism. The prediction indicates that the reaction mechanism can predict the combustion characteristics of the model fuel well. According to the analysis of the reaction path at 900K, it can be seen that at this temperature, 1,3,5-trimethylcyclohexane mainly produces PXCH2D35MCH radical consumption through dehydrogenation; n-propylbenzene generates more A1CHCH2CH3 and A1CH2CHCH3 radical, followed by β dissociation to generate styrene; n-dodecane is mainly consumed through dehydrogenation to generate C12H25 radical, which generates peroxide radical PC12H25O2 through O2 addition reaction. According to the sensitivity analysis at 900K (Figure 2), we can see that the reaction T135MCH + H = PXCH2D35MCH + H2 plays the most important role in the consumption of 1,3,5-trimethylcyclohexane; the reaction NPB + OH = A1CHCH2CH3 + H2O It plays the biggest role in the consumption of n-propylbenzene; the reaction CH3 + O2 = CH2O + OH and the reaction CH3 + CH3 (+ M) = C2H6 (+ M) are the biggest promotion of the consumption of the two groups of fuel components respectively And inhibition reactions. At the same time, the key reaction in the process of n-dodecane consumption P12OOHX2 = PC12H25O2 simultaneously inhibited the oxidation of these two components.
Related results were published on Combustion and Flame [202 (2019) 252-261] by the Institute of Engineering Thermophysics as the first author and corresponding author. This work was supported by the Natural Science Foundation (91541102/51476168), the Ministry of Science and Technology's Key R & D Program (2017YFA0402800), and the German Humboldt Fund Research Cooperation Group. It is the result of a joint research between the Institute of Engineering Thermophysics and the German Aerospace Center.

Fig.1 Curves of oxidation mole fraction of main components of aviation kerosene model fuel, points and lines represent experimental and simulation results, respectively
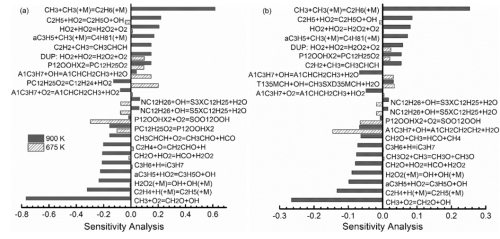
Figure 2 Sensitivity analysis diagram of 1,3,5 trimethylcyclohexane and n-propylbenzene
Adhesive Activated Low Shrinkage Polyester Yarn
Aa Ms Polyester Yarn,Ms Aa Polyester Yarn,Medium Shrinage Polyester Warp Yarn,Medium Shrinage Polyester Weft Yarn
ZHEJIANG GUXIANDAO POLYESTER DOPE DYED YARN CO., LTD , https://www.htpolyesteryarn.com